I am getting questions about prepping brains for the classroom so here are my Top Tips.
There is not much point putting brains into the classroom unless they have been prepped. You just end up with a pink puddle of mush.
My preference is at least 24 hours in 50% alcohol from frozen. They will float so you need to turn them over periodically or fill a container to the brim and put the lid on them to keep them submerged.
Saturated salt solution works well, too, but I am too lazy to be bothered making it when I can just dilute a bit of metho.
Patricia Hugman developed this 1 hour method that she swears by and gave it to me to put on the website. Don’t be stingy with the salt. Really load them up.
https://dissectionconnection.com.au/top-tip-how-to-defrost…/
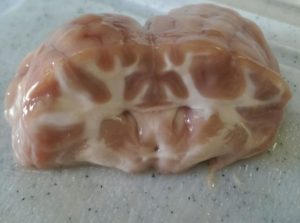
I heard that you can microwave brains to firm them up. This one in the picture has been subjected to 1min 30sec in water in 10 sec blasts. It was ONE brain in a container. If you are going to do more then you will have a bigger container, more water and more brain so you will have to fiddle with the timing a bit.
I do know people that freeze them on a tray and deliver them to the classroom that way so they defrost during the dissection. I haven’t tried it but I can see how it would work.
Good luck! Terrible things, brains.